Fuel for Internal Combustion Engines for
the 21st Century
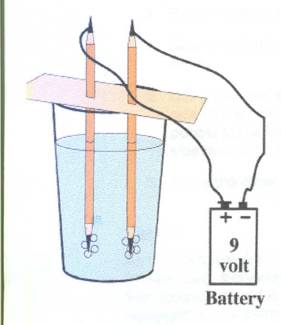
Splitting Water
H20 Hydrolysis
Electricity is “created” when certain chemicals react together. We use chemically made electricity to power many machines from flashlights to a watch or sometimes a car. Yes, there are cars that run on electricity! The devices that store electricity are called batteries. Electricity can also be used to produce chemical changes.
Water is a simple chemical made from two gases – hydrogen and oxygen. Every molecule of water has two atoms of hydrogen for every atom of oxygen. H2O is the chemical formula for a molecule of water.
If an electrical current is passed through water between electrodes (the positive and minus poles of a battery), the water is split into its two parts: oxygen and hydrogen. This process is called electrolysis and is used in industry in many ways, such as making metals like aluminum. If one of the electrodes is a metal, it will become covered or plated with any metal in the solution. This is how objects are silver plated.
TRY THIS!!!
You can use electricity to split water into its two gases – oxygen and hydrogen.
WHAT YOU’LL NEED
· A 9 volt batter
· Two regular number 2 pencils (remove eraser and metal part on the ends)
· Salt
· Thin cardboard
· Electrical Wire
· Small Glass
· Water
WHAT TO DO
1. Sharpen each pencil at both ends.
2. Cut the cardboard to fit over the glass.
3. Push the two pencils into the cardboard, about an inch apart.
4. Dissolve about a teaspoon of salt into the warm water and let sit for a while.
5. Using one piece of the electrical wire, connect one end on the positive side of the battery and the other to the black graphite (the “lead” of the pencil) at the top of the sharpened pencil. Do the same for the negative side connecting it to the second pencil top.
6. Place the other two ends of the pencil into the salted water.
RESULTS
As the electricity from the battery passes through and between the electrodes (the pencils), the water splits into hydrogen and oxygen, which collect as very tiny bubbles around each pencil tip. These bubbles can be collected to provide hydrogen at one electrode and oxygen at the other. Platinum electrodes may also be used for the separation process.
FOR MORE INFORMATION PLEASE CONTACT
The Regional Collaborative for Excellence
in Science Teaching
Project Director
Dr. Jim Roberts
Department of Physics
Denton, Texas 76203-1427